Arkivum is delighted to publish an infographic highlighting the major trends impacting the long-term management of clinical trial data for life sciences organisations. Arkivum’s inaugural TMF survey, ‘TMF Futures: Keeping Data Alive’ was conducted in July 2020, against the backdrop of COVID-19, and is built around the insights and experiences of over 200 life sciences professionals.
You can download the full infographic here
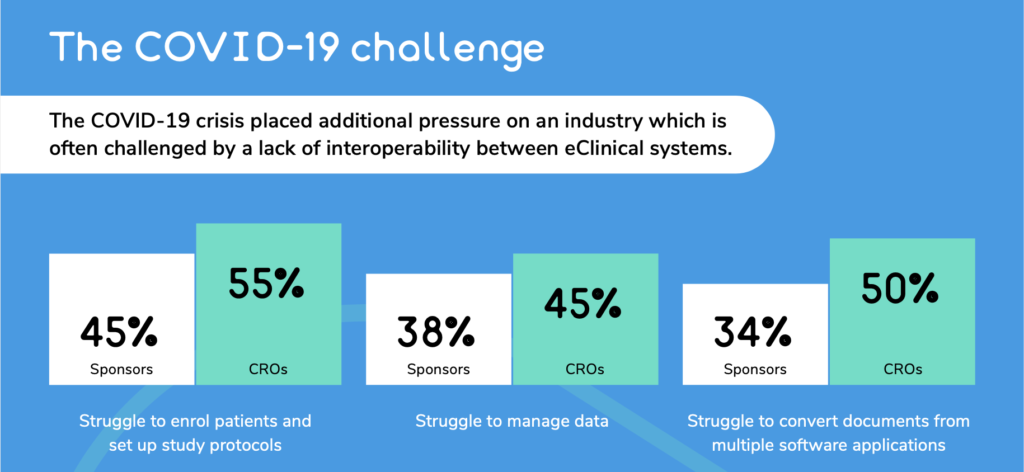
In March 2020 the World Health Organisation (WHO) declared a global pandemic. One area that has been affected are clinical trials, with 74% of individuals in the Life Sciences sector stating that COVID-19 had negatively impacted their ability to deliver on clinical trial objectives.
In response, many organisations have highlighted and accelerated the process of digital transformation that has been underway for some time. Great strides have been made in modernising the clinical trial process with 40% of organisations having transitioned to a purpose-built eTMF application in 2020 compared to only 25% in 2015.
As the industry adapts, 70% of respondents say that the pandemic presents an opportunity for changing the way clinical trials are conducted in the future.
You can download our full infographic with data from our TMF Futures survey here.
Alternatively, for a more in-depth analysis of the data in this infographic, you can also download our free TMF Future- Keeping Data Alive Report.
Suggested reading
23 Sep, 2020
Arkivum report: TMF Futures, Keeping Data Alive
08 Apr, 2020
eTMF archiving myths, misconceptions, and half-truths – here’s what you need to know
01 Dec, 2020