A Risk Based Approach to Long Term Data Integrity of Clinical Data and The Role of CDISC Standards
Regulatory guidelines require Life sciences organisations to adopt a risk-based approach in managing long-term clinical data, but what exactly does this involve when it comes to long-term retention? This blog is based on Arkivum’s Matthew Addis’s presentation and poster at the CDISC and TMF Interchange in Berlin earlier this year, where he addressed this question in detail.
The simple answer is that there is not one thing you can do, there are several. Taking a multifaceted approach is far more effective and limits any risk to the long-term integrity of clinical data. In this case, Matthew identifies four pillars:
- Risk-Based Methodologies
- Aligning with ALCOA++ guidelines
- Digital Safeguarding & Preservation Strategies
- Leveraging best practices
In this blog post, we delve into each of these pillars to understand their significance and role in safeguarding the integrity of clinical data.
A Risk-Based Approach
One of the foundational pillars of ensuring long-term data integrity is adopting a risk-based methodology. This can involve assessing the potential harm and severity of data corruption or loss and then evaluating the probability of this happening. By categorising data based on its importance and vulnerability, organisations can prioritise resources and interventions to mitigate risks. For example, critical data for regulatory compliance or patient safety may require more stringent protection measures.
Aligning with ALCOA++ Guidelines
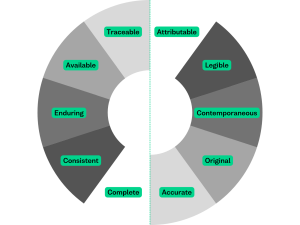
ALCOA++ serves as guiding principles which are frequently used by life sciences organisations when maintaining the integrity of clinical data. When applying ALCOA++ principles, it’s essential to differentiate between live and archived data, as the strategies/approach employed to adhere to these principles may vary.
To provide some examples and context relating to archived data, the guidelines inform us that data must be legible, not only does this support staff being able to access and reuse past data but it also supports a smoother inspection. To support the legible principle an organisation may decide to convert data into long-term preservation formats to guard against obsolescence and ensure data can always be opened and used. Traceable requires any changes to data are recorded. This may prompt organisations to create detailed and persistent audit trails to view past events and reconstruct previous versions of their data. ALCOA++ mentions that data must be enduring and protected against data corruption or loss. Organisations should store multiple copies of their data and introduce regular data integrity checks.
Digital Safeguarding & Preservation Strategies
It is important to remember that there are fundamental differences between data archiving and digital preservation. While data archiving focuses on storing or backing up your data, it is not sufficient for ensuring data remains reusable after 25 years. When introducing a digital safeguarding and preservation strategy it can comprise of three pillars, Technology, Organisation and Resources.
Technology – This looks at the type of system for long-term archiving and preservation. It can also include the range of tools/features that support your archived data. Regular data integrity checks using technologies like checksums help maintain data accuracy. Preservation copies ensure future compatibility by updating data to newer formats. Automation enhances efficiency in handling extensive clinical data volumes, thereby freeing up staff time. Finally, robust access controls protect against unauthorised access.
Organisation – This involves the establishment of processes and procedures. This can include outlining clear roles and responsibilities, ensuring accountability throughout the preservation process. Setting guidelines on data retention, access, and disposal, aligning with regulatory requirements and organisational needs.
Resources – Identifying the necessary support to ensure effective management of digital assets over time. Securing adequate funding and budgeting is essential to sustain preservation initiatives. Skilled personnel, including trained archivists and IT professionals, are crucial for implementing and maintaining preservation strategies.
Leveraging best practices
It must be recognised that the digital preservation community has over 30 years’ experience in ensuring digital content remains accessible and useable over time. They are a valuable asset to highly regulated industries such as life sciences. Therefore, aligning with guidelines and standards from bodies such as CDISC, DPC and NDSA can support your efforts in long-term digital preservation.
Conclusion
In conclusion, safeguarding the long-term integrity of clinical data requires a multifaceted approach, as highlighted in this blog post. Matthew Addis’s presentation at the CDISC and TMF Interchange emphasised the importance of adopting a risk-based methodology, adhering to ALCOA++ principles, implementing digital preservation strategies, and leveraging the experience and expertise of bodies such as DPC. Each of these pillars plays a crucial role in mitigating risks and maintaining the integrity of your clinical data over time.

Anthony Wells
Anthony assumed the role of Product Marketing Manager at Arkivum in 2024, leveraging over a decade of experience of product marketing management in the technology sector. Proficient in developing and executing marketing strategies, Anthony is also experienced in product lifecycle management, from inception through to discontinuation.
Get in touch
Interested in finding out more? Click the link below to arrange a time with one of our experienced team members.
Book a demo