ALCOA+ Principles: The Cornerstone of Data Integrity in Life Sciences
ALCOA+ represents a set of guiding principles for maintaining data integrity. Originally introduced by the US Food and Drug Administration (FDA) in the 1990s, alcoa+ principles have evolved to align with current challenges and demands from the sector.
These principles are frequently directly and indirectly referenced within GxP regulations and guidelines such as the FDA’s 21 CFR Part 11, the EMA’s Guideline on computerised systems and electronic data in clinical trials, EU CTR and the soon to be updated ICH E6 (R3). This integration underscores the important role ALCOA+ plays in maintaining data integrity standards across the life sciences industry.
Below I’ve detailed the nine principles which make up ALCOA+ (sometimes referred to as ALCOA++) and a brief consideration from the data retention perspective. At Arkivum we often find that the principles are strictly adhered to while the data is still in use day-to-day, but approaches are not adapted to the challenges presented when retaining data for decades or longer.
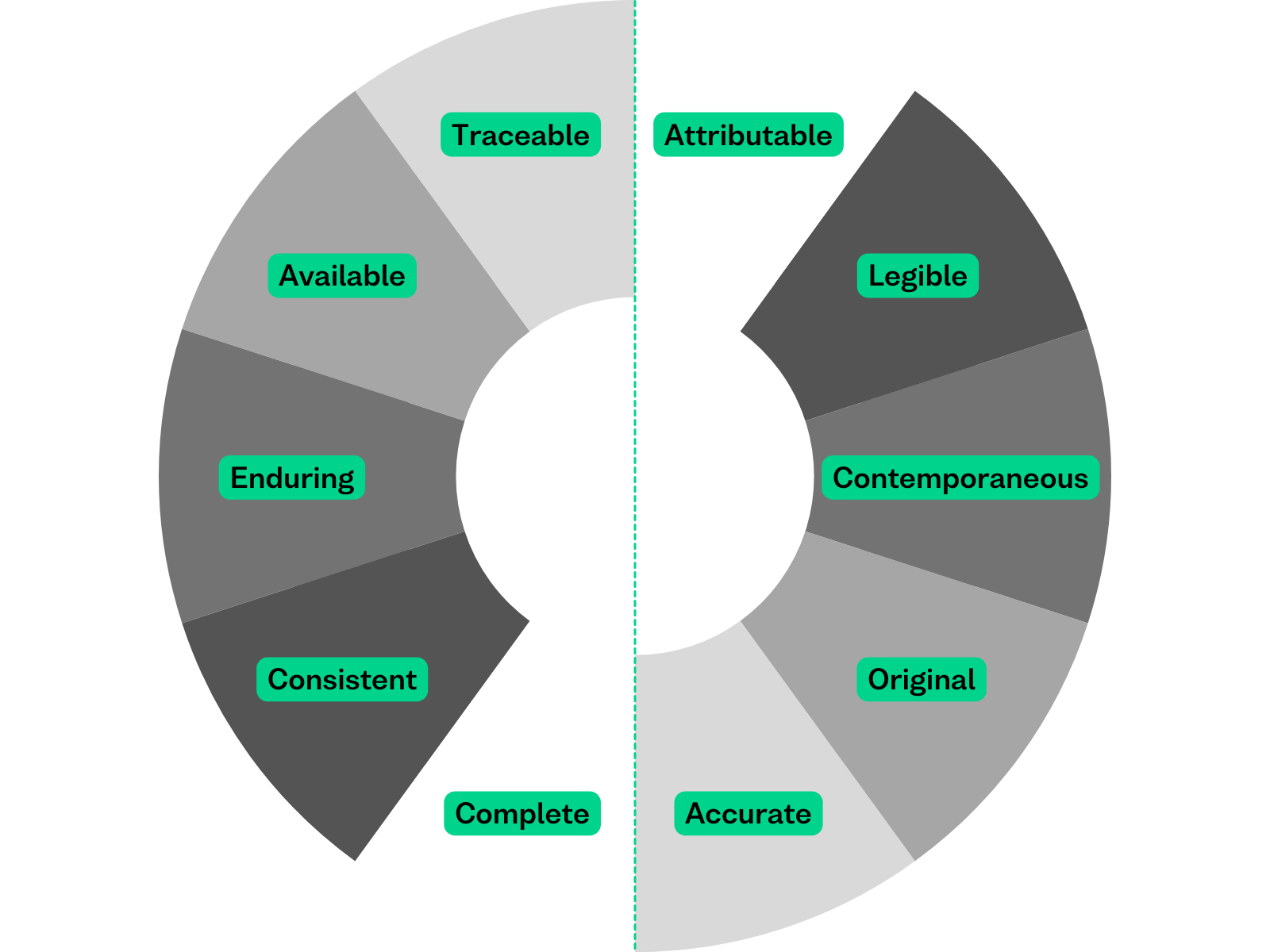
The ALCOA+ Principles
- Attributable: The principle of attributability ensures that every piece of data is traceable to its creator. In pharmaceutical research and development, this means that each experiment, observation, or analysis must be clearly linked to the individual who conducted it. This attribution must be maintained throughout the entire lifecycle of the data, including relevant metadata and audit trails.
- Legible: Legibility refers to the requirement that data must be recorded in a clear, readable, and permanent manner. In the pharmaceutical and life sciences sectors, where data is often generated through complex experiments and clinical trials, legibility is essential for ensuring that information can be accurately interpreted by reviewers and auditors. Long-term legibility can be achieved through the application of digital preservation techniques. Without this, data is at risk of becoming obsolete.
- Contemporaneous: The principle of contemporaneity emphasises that data should be recorded at the time the work is performed. This real-time documentation practice is crucial where timely and accurate data capture is needed. It is important to ensure that an appropriate amount of metadata is being stored to support assertions that data was captured contemporaneously throughout the entire lifecycle.
- Original: Originality requires that the primary source of data or a verified true copy is preserved. This principle ensures the authenticity and integrity of data throughout its lifecycle, from initial data capture through to regulatory submissions and long-term storage. In pharmaceutical quality control and assurance processes, adherence to the originality principle ensures that all testing and validation activities are documented accurately and can be verified during regulatory inspections. Ensuring data is original includes making true copies through preservation techniques such as file format conversion, ensuring there is no loss of data integrity occurs when data is replicated and stored in different locations, and using metadata to record both the origin of data and any subsequent actions done on that data to ensure it remains accessible and usable.
- Accurate: Accuracy mandates that data must be error-free and reflect the true observations or measurements obtained during experiments or clinical trials. The smallest inaccuracies can have significant implications for patient safety and regulatory compliance. By implementing rigorous quality control measures and validation protocols, pharmaceutical companies can verify the accuracy of their data and mitigate the risk of data integrity issues that could jeopardise product quality or regulatory approval. Accuracy includes no loss of data integrity when data is transferred or migrated between systems or into the archive. Accuracy should be recorded in the metadata.
- Complete (+): Completeness requires that all relevant data, including any amendments or modifications, are recorded and retained. This principle ensures that a comprehensive record of all experimental procedures, analytical results, and regulatory submissions is maintained throughout the product lifecycle. Complete includes no loss of data integrity when the data is transferred or migrated between systems. Control is also needed over deletion or alteration of data and any changes to the data should be recorded in the metadata.
- Consistent (+): Consistency dictates that data should be recorded and documented in a standardised manner according to established protocols and procedures. This principle ensures uniformity and comparability of data across different studies, experiments, and manufacturing processes. Consistent includes checks of the metadata and content. Some aspects of consistency can be done through integrity checks, (e.g. checksums) and other file attributes match the metadata.
- Enduring (+): The principle of endurance requires that data must be preserved in a durable medium for the required retention period. In life sciences, where regulatory authorities mandate the retention of records for extended periods to support product safety and efficacy assessments, enduring data storage practices are essential. Enduring includes preventing data corruption or loss using appropriate storage, and safeguarding techniques such automated integrity checks and managing access control appropriately.
- Available (+): Availability mandates that data must be readily accessible for review, audit, and inspection purposes throughout its retention period. This principle supports transparency and accountability by ensuring that regulatory authorities and other stakeholders can access and evaluate data as needed to verify compliance with regulatory requirements and quality standards. Available data needs to be held on storage that enables easy access (for authorised personal) as well as digital preservation. Metadata is needed to support search and discovery across large datasets. Control is needed over access and to record any access in an audit trail.
Summary
The ALCOA+ principles form a comprehensive set of principles that is essential for maintaining data integrity in the life sciences industry. From ensuring data is attributable and accurate to guaranteeing its consistency and availability, the nine principles cover all aspects of data management throughout the entire lifecycle of pharmaceutical products. By adhering to ALCOA+, life sciences organisations can not only meet regulatory requirements but also enhance the quality and reliability of their research, manufacturing, and testing processes.

Anthony Wells
Anthony assumed the role of Product Marketing Manager at Arkivum in 2024, leveraging over a decade of experience of product marketing management in the technology sector. Proficient in developing and executing marketing strategies, Anthony is also experienced in product lifecycle management, from inception through to discontinuation.
Get in touch
Interested in finding out more? Click the link below to arrange a time with one of our experienced team members.
Book a demo