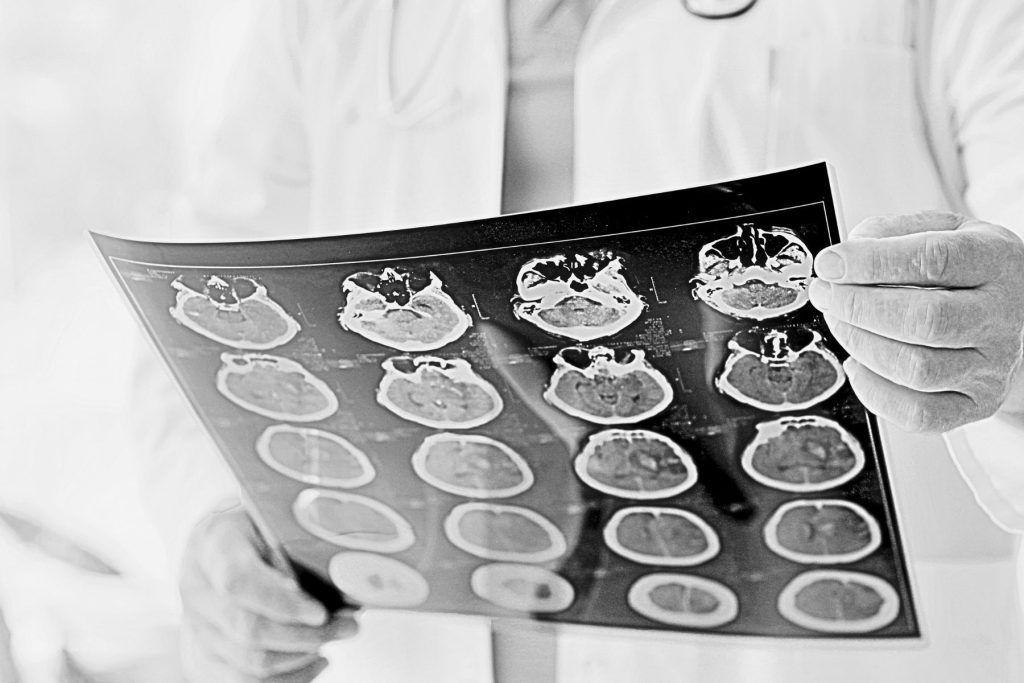
The Only Validated GCP Archiving and Preservation Solution
Data Retention: Compliance is complex. Complying doesn’t have to be.
Clinical trial sponsors face a complex regulatory landscape, especially when it comes to data retention. Regulations such as EU CTR state that your eTMF needs to be retained for 25 years or more. Data must be accessible and legible throughout that period.
And that’s before you’ve considered the long-term needs for other clinical data, such as eCRF, eCOA and ePRO.
As part of a risk-based approach it is essential that you select a GCP compliant archive and preservation solution, enabling you to maintain data integrity in line with ALCOA+ throughout the entire clinical data lifecycle. This supports compliance with regulations and guidelines such as EU CTR, FDA CFR 21 Part 11 and ICH E6 R3.
“The content of the clinical trial master file shall be archived in a way that ensures that it is readily available and accessible, upon request, to the competent authorities.”
EU Regulation 536 of the European Parliament

Centralised digital preservation for long-term GCP compliance
Leaving data in an eTMF solution, eClinical system or even a file sharing platform such as SharePoint is not viable long-term archiving solution. These systems are not designed to maintain data integrity for decades or longer and can leave your organisation at risk of critical inspection findings.
To guarantee data endures and remains legible, sponsors must actively maintain and preserve their data throughout the entire retention period
Archiving all long-term clinical records and data in a single repository enables:
- Active digital safeguarding and preservation to maintain data integrity in line with ALCOA+ forever
- Adherence to EU CTR, FDA CFR 21 Part 11 and ICH E6 R3
- Easily search and access clinical records and data, supporting inspection readiness
- Manage audited user access and regulatory demands
- Cost-effective long-term solution which avoids vendor lock-in
Find out more about the Arkivum solution here
GCP Archive Storage and Data Integrity
Arkivum is the only validated GCP archiving and preservation solution. Our retention system supports clinical trial sponsors, sites and CROs to ensure that their records and data are inspection-ready and retained in line with the ALCOA+ principles.
Below are some of the key features supporting life sciences organisations within clinical trials. Click on the links below to find out more.
Preservation of Clinical Trial Records and Data
At Arkivum, we don’t just archive data; we preserve it. Recognising that the software and hardware of today may not be accessible in 25 years’ time, our solution remains ahead of the curve, guaranteeing that your valuable GCP data remains both legible and human-readable in the future.

Regulatory Tools to Manage Retention Requirements
Arkivum simplifies compliance. Our solution ensures your data is always inspection-ready, featuring robust audit trails and full data encryption. Managing highly regulated long-term data does not have to be difficult, Arkivum’s validated archiving and preservation solution aligns with EU CTR, ensuring the integrity of your data for as long as you need it.
Data Migration Service
As part of Arkivum’s fully validated migration service, we collaborate directly with CROs and sponsors to ensure seamless and effective data migration, minimising disruption to our clients. Once the data is archived, automatic data integrity checks ensure that all data is complete, providing peace of mind.
eBook: A guide to preserving essential GCP records and data
Download our free guide on how and where to get started with your GCP data archiving and preservation. Includes compliance with key regulations and the long-term alignment to the ALCOA+ principles.
Partner with Arkivum
Are you from a CRO, GCP consultancy or eClinical solution provider?
Arkivum work with a range of partners within the clinical trial sector, supporting the application of good clinical practice. If you’re interested in collaborating with Arkivum, please explore our partner page.
eTMF and GCP Compliance FAQs
Why can’t I leave my data in an eTMF solution at the end of a study phase?
While it is possible to leave data in an eTMF system at the end of trial, there are several reasons why Arkivum would strongly recommend migrating the data to a dedicated digital archive.
Firstly, eTMF systems are not designed to archive data for long periods of time, and do not have the capability to safeguard and preserve digital content. The longer that it is kept within the source system, the greater at risk that data is.
Secondly, the longer that data is kept within the source system, the harder and more complex the data migration process will be. As staff move onto other projects or churn, it will become the responsibility of those unfamiliar with the data to migrate it.
Finally, Arkivum typically find that it is much more cost effective for customers to move their long-term data into a centralised repository. An added benefit of taking this approach is that it avoids vendor lock in and data becoming stuck in disparate and siloed systems.
Can I archive clinical data with Arkivum?
Yes, it is possible to archive any clinical data within the Arkivum solution. This includes (but not limited to), eCRF, eCTD, ePRO and more.
Which eTMF solution providers does your solution support?
Arkivum is experienced at working with a range of different eTMF and eClinical solutions. Where direct integrations do not exist, we can work with you as part of the migration service to extract and prepare your data for archive, regardless of source system.
Does the Arkivum solution align to the TMF reference model?
Yes, the Arkivum solution supports the TMF reference model. Users can use whatever structure within the archive as required, including both a standard or bespoke application of the reference model. It is possible for one customer to also adopt multiple structures for their data within the system, for example if a sponsor has multiple studies, from multiples trials, all in different structures.
Do you work directly with CROs?
Yes, Arkivum typically works directly with CROs as part of our migration service. Depending on the arrangement, Arkivum can work directly with your CRO partners to receive end-of-study data. As part of that process, we also quality-check all records to ensure completeness and accuracy of the files and records.
How does the Arkivum solution support inspection readiness?
The Arkivum solution is live archive, not a locked system. This means that data can be easily accessed and searched, enabling life sciences organisations to easily make requested data available. In some instances, customers may even want to provide inspectors with a bespoke login, enabling them to review and search relevant documentation as required.
In addition to this, by maintaining all of your long-term and quality checked records and data in a single repository, customers can have peace of mind that is it both inspection prepared and inspection ready.
Does the Arkivum solution adhere to regulations such as EU CTR and the FDA CFR 21 Part 11?
Yes, the Arkivum solution has been developed to support life sciences organisations to adhere to retention regulations and guidance. This includes EU CTR (EU Regulation 536/2014) which requires the retention of the TMF for at least 25 years, the FDA’s CFR 21 Part 11 and ICH E6 R3.
The solution has also been mapped to industry best practice guidelines such as the ALCOA++ principles, which are included with the EU’s Guidelines for Computerised Systems.
Do you only archive eTMF records?
No, the Arkivum solution can and is used to archive a range of GxP and non-GxP data. Within the clinical arena this could include but not limited to eCRF, eCTD and ePRO datasets.
What validation testing do you do?
Arkivum conducts computer system validation (CSV) which includes testing and confirming that the Arkivum solution meets documented security requirements. Arkivum also conducts penetration testing on the solution.
Do you have validation documentation?
Where required, Arkivum provides a validation pack (which includes a requirements traceability matrix, test plan, test scripts, and validation summary report), a 21 CFR Part 11 compliance assessment, a penetration testing report, and the impact assessment for GxP Data Integrity, Confidentiality, Privacy.