Managing your eTMF archive: an effective approach to digital preservation
Last year we published a post on good electronic Trial Master File (or eTMF) archiving practices and what you needed to know. Given the time that’s passed, I thought it would be a good opportunity to revisit the topic and provide a fresh overview of what effective eTMF archiving should look like.
Before I begin, it’s worth mentioning that many of the approaches outlined in this post are applicable to any data that needs to be retained in heavily regulated industries.
Why do you need to preserve your eTMF?
Before we explore what good practice looks like, I think it is important to provide the context for why effective eTMF preservation is required. Understanding the complete requirements to adhere to regulations, and the subsequent challenges that they create helps to fully realise what solution is required.
Regulations on the preservation and archiving of the eTMF are constantly evolving. To give a couple of examples, in recent years we’ve seen moves by the EMA to stipulate that the eTMF must be readily accessible for up to 25 years and in 2014, the MHRA also ruled that a deficiency in the TMF could be classed as a critical finding in an inspection.
Given this prevailing wind, it is not too hard to imagine that similar bodies such as the FDA will look to adopt similar guidelines in the future. Digital or e-clinical applications and archives are becoming more prevalent, and guidelines have and will continue to evolve to address these changes.
Looking specifically at the eTMF, there are many guidelines across the various bodies. Below I have sought to summarise some of the key stipulations in relation to archiving and preservation;
- The retention of the data for up to and in some cases beyond 25 years
- Ensuring the eTMF is securely stored, and that access is only provided to those who need to access it. This could be someone within the organisation, or an inspector
- The eTMF should be stored in a complete state, and crucially should be stored in such as a way that it is possible to fully rebuild if required
- There should be a complete audit trail of who has accessed the file
- And the data should be archived in a way in which it will not deteriorate. This would include ensuring that a strategy is in place to avoid file format obsolescence and data loss or corruption.
We have found in our research that many sponsors are content with their CRO or eTMF solution provider simply backing up these files. Or alternatively placing them on a single cloud storage system. This type of approach is not sufficient, and puts the eTMF at risk of damage or loss.
Instead, an effective preservation strategy is required, and one which must ensure that the right people, processes and technology are being utilised.
When should I plan to archive my eTMF?
The recent findings from our 2020 TMF Futures report found that there were a range of approaches taken to when to start thinking about the long-term storage of the eTMF.
From the chart below you can see that there is a broad spread of when archiving comes into consideration for sponsors. In reality this is indicative of the fact that up until registration, it is still possible to plan to archive your eTMF.
What phase do you start planning for the archival of your TMF?
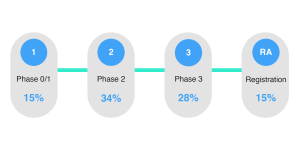
Yet in our experience, and the work that we do with our customers, we’d highly recommend planning your preservation strategy in phase 1. Effective preservation is challenging and ensuring that the right elements are in place can go a long way to ensuring compliance.
Preserving your eTMF archive
We’ve talked about why you need to archive your eTMF, outlined the challenges that can entail and also covered when to begin planning, but we have yet to explore what an effective preservation approach looks like.
I mentioned earlier in this blog post that effective preservation includes ensuring you have the right people, processes and technology in place to succeed. What I mean by this is having the right expertise, following and managing the right processes, supported by the right and robust tools.
We would highly recommend any organisation, life sciences or otherwise, who are looking at effective long-term data management to take note of both ALCOA+ and the FAIR data management principles. These best practice approaches provide a fantastic overview of what you need to consider when planning your data management approach (and I have provided links to further information on each). They help outline what to consider when planning how your data will be secure, preserved and accessible for however long you need to store it.
Automated, robust and fully validated technology solutions can then ensure that these principles are realised in the real world.
Here are a couple of examples of what a preservation tool will provide:
- Data can be stored in multiple locations by one solution to protect against data loss
- Regular automated checks of each file to quickly identify issues of data corruption
- Audit logs can track who has accessed the files and when
- File normalisation will overcome file format obsolescence, by maintaining an up-to-date version of your file, no matter how often formats change
- Secure access can be provided to the right documents when an inspector needs them, no matter how long after marketing approval.
These are just some examples of what a robust preservation solution can provide, and how it would support records and archiving staff within pharma and life sciences to follow best practice guidance.
In Summary
Sadly, there is no silver bullet to effective digital preservation. There is though plenty of support available.
If we look at the evolution of regulations over the last couple years, they are only heading in one direction. And while this conversation has focused on this regulatory angle, there are in fact many opportunities beyond compliance in regard to the long-term management of your clinical trial data and the eTMF. I won’t say any more here, but if you are interested, our recent blog post covered this very topic.
As the clinical trial process looks to embrace a growing number of digital technologies, sponsors and CROs need to find smarter ways in which to manage their data. This includes ensuring that an effective preservation and archiving strategy is in place.
If you would like to speak to one of the team about your preservation challenges, please contact us here.

Tom Lynam
Tom is the Marketing Director at Arkivum. He joined the business in January 2020 tasked with driving new business growth and building the brand into new sectors such as Pharmaceutical and Life Sciences. He has over 12 years’ experience in several diverse marketing leadership roles across technology and professional services organisations.
Get in touch
Interested in finding out more? Click the link below to arrange a time with one of our experienced team members.
Book a demo