When is the right time to archive your eTMF?
The archival of eTMFs has been the focus of much discussion since regulators began to increase their focus on the topic.
Both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) provided updated guidance on the topic in 2007 and 2018 respectively. We won’t explore these regulations in more detail in this post, but further information on the requirements can be found in our eTMF Archiving Preservation Guide.
Lack of clarity
Both the FDA and EMA regulations are less prescriptive however when it comes to planning for archival. Given that getting a product through development and trials can be a decades-long effort, this offers a lot of leeway.
Some further guidance has been offered by bodies such as The Health Sciences Records and Archives Association (HSRAA), which stated that:
“There should be documented procedures in place which define the processes for archiving of trial materials, including checks for completeness, timeframes for submission to archive, delegation of responsibility…. It is recommended that all trial materials, not already archived, are archived promptly on completion of the trial – or as immediately as possible thereafter – as defined by the sponsor.”
Again, there is no prescriptive timings and this is resulting in inconsistency and uncertainty across the industry.
In Arkivum’s research last year: TMF Futures, Keeping Data Alive the data shows that there are a range of approaches taken across the life sciences industry when deciding the stage of a clinical trial to start planning the archival of the eTMF.
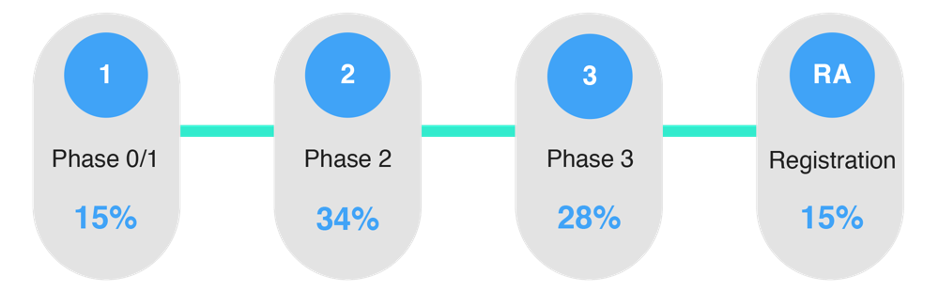
It is clear that there is a lack of consensus among sponsors.
The risk, and the answer
The TMF acts as a custodian of invaluable data and can offer a narrative of a product’s progress through each trial phase and to market. A good eTMF is designed to tell the story of how studies are structured, supporting decision-making and evidencing how certain stages are reached when auditors and investigators begin to view the information.
Correct archival and active maintenance of the eTMF ensures this ‘story’ is told and understood accurately across the lifecycle of a product. It is fairly common for sponsors not to archive for the first few years after registration, however, inspections are more likely to take place during this timeframe than any other. It is also a relatively common misconception held by sponsors that eTMF vendors and CROs provide long-term storage of the data, and while they may ‘store’ the data, this is different from the active maintenance and preservation that regulations now require in archival.
In both instances, this can leave a white space where the sponsor is underprepared for auditors and potentially not compliant with archival regulations. Having the eTMF ready for archival, and immediately archived following registration, completely negates this risk.
Based on Arkivum’s experience working with a variety of customers across the sector, it is highly recommended to begin planning a preservation strategy from the ground up at the pre-clinical phase to create efficiency and a more robust archive.
All data should be archived irrelevant of the progress or success of development and trials as it may support future innovation and development efforts. This approach is vital in the biotech market – where the trend is for smaller virtual organisations to develop a product with no intention of marketing it, but to sell it to a larger player. If they already have the eTMF packaged up, archived and ready to sell-on, this makes the product more appealing.
The impact of COVID-19 on clinical trials has also been notable. Virtual or decentralised trials have quickly gained prominence in response to the disruption caused by lockdowns that limited movement and restricted access to trial sites. Regional approaches remain incongruous, but the pressure to progress with trials has remained, this has led to trial processes that are less linear and more agile and iterative with different models running concurrently in some instances.
In these instances, planning archival, and how the eTMF will be collated and presented, as early as possible offers continuing returns for sponsors and is an essential element of a more mature approach to long-term data management and preservation.
A new approach
Implementing an archival process early, training the responsible staff on its use and adopting protocols that ensure regulatory compliance and data integrity can negate risk and create efficiency in a trial. Early planning can also assist in the long-term management of documents once the study has closed.
Having the right system in place from the first phase of a trial ensures that sponsors stay in control and manage their data more effectively from the beginning. This ensures effective preservation of clinical data and provides a central piece of the compliance puzzle.
Arkivum provides validated digital archiving support and software for document tracking. Our experienced team is ready to protect your critical data and guarantee that it is accessible, secure, reliable, and immutable, now and in the future.
To learn more about how we deliver complete data integrity and data preservation for the long term, book a demo today.

Whitney Armstrong
Whitney is the Marketing Executive at Arkivum. She joined the business in 2020 and is responsible for supporting marketing campaigns and activities targeting key sectors. Whitney has over 5 years' experience of delivering and supporting marketing strategy for technology brands.
Get in touch
Interested in finding out more? Click the link below to arrange a time with one of our experienced team members.
Book a demo