eCTD Archiving for Regulatory Submissions: Everything You Need to Know
As regulatory requirements for clinical trial documentation evolve, organisations face growing challenges to ensure the long-term retention and accessibility of electronic Common Technical Documents (eCTDs) for regulatory submissions. With increasing regulatory scrutiny and eCTD version 4.0 adoption on the horizon, a robust archiving strategy is more essential than ever.
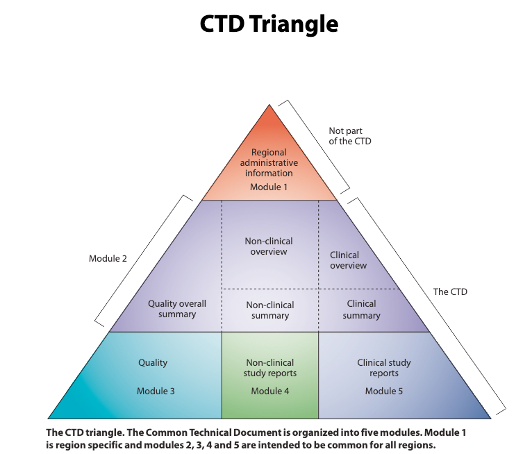
The 5 Key Modules of eCTD for Efficient Regulatory Submissions
An eCTD, or electronic Common Technical Document, is the standardised format for submitting study documentation and data to regulatory authorities worldwide, including the FDA, EMA, and MHRA. The structure of an eCTD standardises pharmaceutical regulatory submissions. It includes region-specific content in Module 1, which varies by regulatory authority (e.g., FDA, EMA), and standardised content in Modules 2 to 5, covering summaries, quality, nonclinical, and clinical study reports.
eCTDs can contain various data types, such as datasets from Electronic Data Capture (EDC) systems and their statistical analyses, following standards like Study Data Tabulation Model (SDTM) and Analysis Data Model (ADaM) from CDISC.
During submission review, regulatory authorities might request additional navigation and indexing tools, like study tagging files, to make the assessment more efficient. More information on eCTDs can be found on the FDA website.
Understanding GxP Data Retention Challenges
Managing evolving regulatory submissions poses significant data retention challenges. Each dossier consists of multiple sequences that change over time, making it difficult to maintain a current and comprehensive view of the structure.
Adding to this complexity, eCTDs may include various content types beyond documents, such as embedded datasets and statistical analyses. Since specialised applications are necessary for viewing and managing eCTDs, organisations must carefully evaluate whether their retention approaches will support future access needs and maintain usability over time.
Managing data across multiple systems presents additional challenges for long-term retention. Essential records span a broad range of documents and records under ICH E6 (R3), consolidating these records into a single archive simplifies compliance and reduces the risks of data fragmentation and loss over time.
eCTD Retention Compliance: Essential Regulatory Requirements
Guidelines such as ICH E6 (R3) clearly define essential records, including eCTDs, as critical for verifying trial results and ensuring compliance with Good Clinical Practice (GCP). Essential records must be securely retained, readily accessible, and maintain data integrity for at least 25 years, according to EU Clinical Trial Regulation (EU CTR) guidelines.
In addition to the eCTD itself, retention obligations extend to validation records for eCTD systems, staff training documentation, and metadata that ensures traceability. Furthermore, organisations must also comply with ALCOA+ principles, which emphasise that data must be Attributable, Legible, Contemporaneous, Original, and Accurate, while ensuring completeness, consistency, and durability.
Key Approaches to eCTD Archiving
There are several approaches to eCTD retention, each with its advantages and challenges:
1. Keeping eCTDs in a Live System (e.g., RIM Systems)
- Pros: Provides easy access, supports built-in eCTD viewers, and simplifies compliance during inspections.
- Cons: Systems may not be designed for long-term retention, necessitating costly migrations as software evolves.
2. Exporting eCTDs to General Storage (e.g., SharePoint, File Repositories)
- Pros: Reduces vendor lock-in and allows for archiving alongside other clinical records.
- Cons: Lacks built-in eCTD navigation tools, which can make retrieval and usability more challenging.
3. Dedicated Digital Preservation and Archiving Solutions
- Pros: Ensures data integrity, enables consistent metadata management, and facilitates regulatory compliance.
- Cons: May require additional configuration to meet eCTD-specific viewing and navigation needs.
Please refer to our blog on document storage options for more information on this.
Why Digital Preservation is Crucial for eCTD Archiving Success
Unlike traditional archiving, which often treats digital records as static assets, digital preservation is an active approach that ensures long-term usability. Key components include:
- Metadata Extraction & Standardisation: Maintaining regulatory metadata for indexing and searchability.
- Format Migration Strategies: Supporting eCTD version upgrades (e.g., v3.2.2 to v4.0).
- Data Integrity Verification: Conducting regular checks to ensure no corruption or unauthorised modifications.
A well-implemented digital preservation strategy helps organisations mitigate risks related to data loss, obsolescence, and compliance failures.
The Future of eCTD Archiving: Ensuring Compliance and Data Integrity
As regulatory landscapes shift and eCTD version 4.0 gains adoption, organisations must future-proof their archiving strategies. The ability to search, navigate, and access eCTD content alongside other clinical records in a unified system will be crucial for maintaining compliance and efficiency.
For organisations exploring eCTD archiving solutions, evaluating systems based on their ability to support long-term data integrity, accessibility, and regulatory compliance (ALCOA+, ICH E6 R3, EU CTR) will be essential. By taking a proactive approach to digital preservation, regulatory teams can ensure that their eCTD archives remain reliable and usable for as long as they are needed.
Watch Arkivum’s CTO Matthew Addis discuss eCTD Archiving & Digital Preservation here

Anthony Wells
Anthony assumed the role of Product Marketing Manager at Arkivum in 2024, leveraging over a decade of experience of product marketing management in the technology sector. Proficient in developing and executing marketing strategies, Anthony is also experienced in product lifecycle management, from inception through to discontinuation.
Get in touch
Interested in finding out more? Click the link below to arrange a time with one of our experienced team members.
Book a demo