What Does ALCOA Stand For?
If you work in clinical research, pharmaceuticals, healthcare, or regulated industries, you’ve likely come across the term ALCOA+. But before diving into the “plus,” it’s important to understand the basics. So, what does ALCOA stand for, and why is it so critical for data integrity?
ALCOA is a widely accepted framework used to ensure data quality, reliability, and compliance with regulatory standards such as FDA, EMA, and ICH guidelines. Over time, ALCOA evolved into ALCOA+, adding more rigor to data integrity practices in modern digital environments.
Origin of ALCOA Principles
The ALCOA acronym originated from regulatory expectations around Good Manufacturing Practice (GMP) and Good Clinical Practice (GCP). Regulatory authorities needed a simple but effective way to define what “good data” looks like.
Initially introduced by the FDA, ALCOA became the foundation for data integrity across life sciences. As systems evolved from paper-based to electronic, regulators expanded the framework, leading to the development of ALCOA+.
What are the ALCOA Principles?
ALCOA is essentially five core principles:
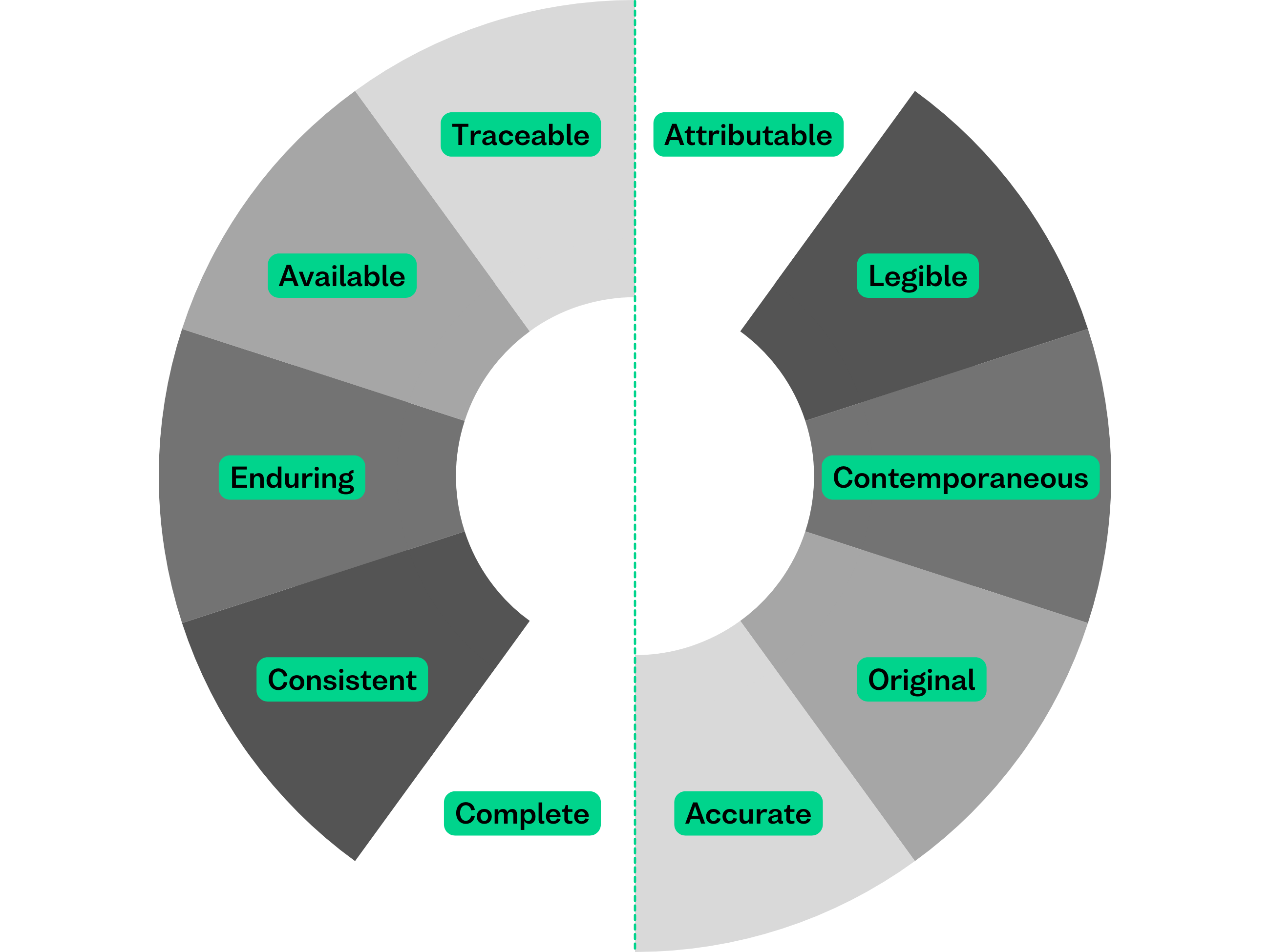
Attribute
Data must be attributable, meaning it should be clear who recorded the data, when it was recorded, and where it came from. This ensures accountability and traceability. In electronic systems, this is often achieved through system-generated metadata, such as user IDs, timestamps and audit trails.
Legible
All data must be legible and readable throughout its lifecycle. Whether paper or electronic, data should be understandable to anyone reviewing it, including auditors and inspectors.
Contemporaneous
Data should be recorded at the time the activity occurs, not hours or days later. Contemporaneous recording reduces errors and improves data accuracy.
Original
Data must be original, or a verified true copy. This means source data should be preserved, whether it’s a paper record or an electronic entry. For electronic records, checksums help demonstrate that original data and its metadata remain unaltered over time, supporting the ALCOA principle of Original.
Accurate
Data must be accurate, reflecting the true observation or result. Errors, inconsistencies, or unexplained changes undermine data integrity.
What’s the Difference Between ALCOA and ALCOA+?
While ALCOA established the foundation, modern regulatory expectations required additional safeguards. This led to ALCOA+, which builds upon the original principles.
Complete
All data must be complete, including any repeat measurements, corrections, or failed results. Missing data can be just as problematic as incorrect data. Ongoing data integrity checks and Checksums can support this.
Consistent
Data should be consistent across systems and over time. This includes consistent timestamps, formats, and sequences of events.
Enduring
Data must be enduring, meaning it is recorded in a durable medium that preserves it for the entire retention period without degradation. This would involve storing long-term data in a fit for purpose archiving and preservation system.
Available
Data should be available and easily retrievable for review, audits, and inspections at any time during its lifecycle.
Together, these additions transform ALCOA into ALCOA+, the gold standard for data integrity today.
Final Thoughts
Following ALCOA+ guidelines more broadly makes it significantly easier for organisations to align with regulatory expectations, whether requirements come from the FDA, EMA, or MHRA. While each authority publishes its own guidance, regulators consistently expect data to be reliable, traceable, complete, and maintained throughout its lifecycle. By applying ALCOA+ principles, organisations establish a common data integrity framework that supports compliance across regions and demonstrates that records are attributable, contemporaneous, accurate, complete, and readily available for regulatory review. Whether you’re managing clinical trials, regulatory submissions, or quality systems, understanding and applying ALCOA+ is essential.
If you would like to learn more about ALCOA+ principles we will be holding a live webinar on 26th February 2026, registration is now open!
Annabel Allum
Annabel is a Marketing Executive at Arkivum and joined the business in 2022. She is responsible for managing various operational marketing activities including email, CRM, website management and campaign support.
Get in touch
Interested in finding out more? Click the link below to arrange a time with one of our experienced team members.
Book a demo