What to plan for when transferring your TMF?
Last month, we explored what to do before receiving data from your CRO at the end of the trial and you know exactly what to do when you get your data back. You have a solution that offers preservation, accessibility and discoverability …as well as being validated to ensure you meet all the requirements…perfect!
Now you just have to GET the data from your CRO.
This isn’t as easy as it sounds.
Firstly, you need to move the data from its original location under the ownership of the CRO and move it to its final location so that you can accept accountability.
Secondly, you need to do this whilst retaining data integrity and providing evidence of chain of custody.
Thirdly, you need to do the above whilst balancing preparations for you next, or ongoing clinical studies.
TMF Migration
To do this it should all start with a plan.
In the last post we referenced needing a plan in place for accepting the data, and now we’re going to explore what that plan should look like in more detail.
Take the time to look at each and every step of the process, which areas could cause the biggest risk and the checks you’ll have in place to identify problems before they become too big an issue.
A basic plan will look something like this:
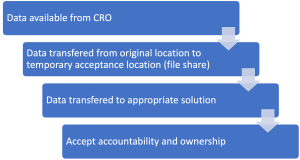
Each transfer creates risk so you will probably want to carry out checks when moving data between each location:
1 – CRO to a temporary location (file share): Rather than just focusing on the data size, understand the number of files and folders which comprise the data. Perform the check after transfer to make sure everything came across.
2- Temporary location to solution: Produce a second inventory and compare against the original.
3- Final solution: Perform a sample quality check (QC) to do a manual user review that the data you expect is what you see. For example, if you open Dr Clark’s CV and it has a date of 20th Jan 2022 on it, does the correct document open or has it been mixed up with another?
You should document this plan along with the checks you will carry out and provide the documentation of each step. This will provide you with evidence of the actions you’ve done should you be audited.
Accepting Accountability and Ownership
You’ll see from the above there are quite a few steps involved and risk associated with each. For this reason, I would never advise anyone to take complete ownership and accountability of the data until you are certain you have everything you need and it’s in its final storage location.
Your CRO can help by providing you with inventory lists and evidence of QC from their end which will help you in the migration process. However, getting your CRO to retain the data until the final stage is vital.
Firstly, it ensures there is still clear ownership of the data, but also acts as risk mitigation if anything were to happen. There is always the case where the process goes through without any issues but while doing the sample QC you spot mismatches – this could be from the original data source and would need to be investigated.
TMF Migration Services
Here at Arkivum we offer a data migration service with every transfer made into Arkivum’s solution. This includes a migration plan and report, evidence of checks at each stage and deliverables of all the evidence for your records.
Our service helps to take away the stress and concerns normally found with migrations and allows you to concentrate on your normal day-to-day tasks, which don’t stop just because you finished one study.
If you’d like to find out any more about migration of data, please reach out to us. We would be more than happy to discuss our services.
Alternatively, please read the next blog in this 3-part series.

Rob Jones
Rob joined Arkivum in 2021 from Phlexglobal, where he spent nearly 3 years supporting the global sales team in promoting TMF best practice and Phlexglobal’s technology. He has worked across multiple departments such as customer success, delivery and onboarding. He has dedicated his career to the field of Life Sciences and has spent his time working for CRO’s, Sponsors and Technology Vendors.
Get in touch
Interested in finding out more? Click the link below to arrange a time with one of our experienced team members.
Book a demo